Search Thermo Fisher Scientific
Foxp3 Overview and Reagents
What is Foxp3?
Foxp3 is the “master transcriptional regulator” for CD4+regulatory T cells (Tregs) [1,2]. Naïve and memory CD4+ T cells can be differentiated into Tregs with induced expression of the Foxp3 gene [3]. Mutation in the Foxp3 gene results in hyperactive T cells and production of pro-inflammatory cytokines [1,2]. Foxp3 protein is used a main identification marker for Treg cells [4].
Foxp3/transcription factor staining buffer set (Cat. No. 00-5523-00) is recommended for detecting Foxp3 proteins. This is a buffer kit containing fix and perm reagents that maximize Foxp3 protein detection and minimize the effect on surface staining. This kit can be used on staining other nuclear proteins.
Identification of T Regulatory Cells with Foxp3
Tregs can be identified as CD4+ Foxp3+ and CD25+Foxp3+ cells in mouse spleen and human PBMCs, respectively [4]. There is a correlation in expression between the CD4+ CD25+ cells and the Foxp3+ Tregs. Downregulation of Foxp3 expression causes Tregs to lose suppressor functions and express some functions of conventional effector Th1, Th2, Th17, and TFH cell subsets. The key causes of this loss of Foxp3 expression include inflammatory environments with high levels of cytokines that are normally involved in the induction of effector T cells, such as IL-6 and IFN gamma [5]. Therefore, Foxp3 protein must be stained for functional Tregs.

Figure 1. Analysis of Foxp3 expression in the CD4+ Treg cell population reveals positive population. C57BL/6 mouse splenocytes were stained intracellularly using the Foxp3/Transcription Factor Staining Buffer Set (Cat. No. 00-5523-00) and protocol, with CD4 Monoclonal Antibody, APC (Cat. No. 17-0042-82), and 0.5 µg of Rat IgG2a kappa Isotype Control, PE (Cat. No. 12-4321-82) (left) or 0.5 µg of Foxp3 Monoclonal Antibody, PE (right). Cells in the lymphocyte gate were used for analysis.
Figure 2. Gating for Foxp3+ Tregs with CD4, CD25, and Foxp3. (Top) BALB/c splenocytes were stained with Anti-Mouse CD4 FITC, Anti-Mouse CD25 APC, and Anti-Mouse/Rat Foxp3 PE. Tregs are identified in the upper-right quadrants as CD4+Foxp3+ cells (right) and CD25+Foxp3+ cells (left). (Bottom) Staining of normal human peripheral blood cells with FITC anti-human CD4 (OKT4) (left) or APC anti-human CD25 (BC96) (right) followed by RBC lysis and intracellular staining using the Whole Blood Foxp3 Staining kit (Cat. No. 88-8996-40). Cells in the lymphocyte gate were used for analysis.
Foxp3 protein is difficult to stain since it is not very abundant, and it is an intracellular protein. The figure below shows Foxp3 protein functional domains and the location of epitopes recognized by Foxp3 antibodies. Clone choice is important for Foxp3 protein detection [6]. For optimal detection of Foxp3 protein, it is important to have optimal antibody titrations, optimized fix and perm buffers, and include cells known to be negative for the marker.
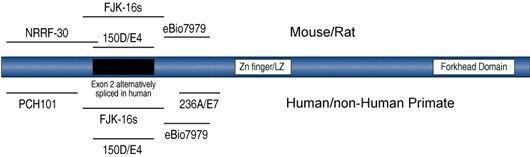
Figure 3. Schematic representation of the Foxp3 protein and the location of the epitopes reactive with antibodies. Foxp3 contains a Zn finger/Leucine zipper and a conserved Forkhead Domain. In addition, in human Tregs, Exon 2 may be alternatively spliced (black box). Currently there is no evidence for alternative splicing in mouse Foxp3. The clone names of Foxp3 antibodies are indicated next to the fragment of Foxp3 where they have been mapped.
Immunohistochemistry of Foxp3 in T regulatory cells
Foxp3 is also expressed by tumor cells. It has been used recently as a biomarker and a prognostic factor for malignant human tumors [7]. Immunohistological staining of endogenous Foxp3 protein can be viewed (in both frozen and paraffin sections) in multiple tissues including spleen, breast, melanoma, and others.
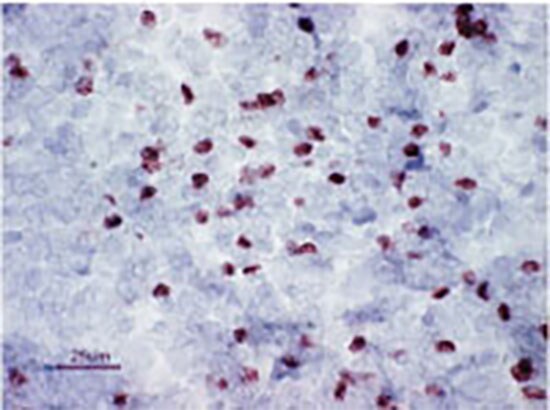
Figure 4. C57BI/6 mouse spleen staining. Staining of C57Bl/6 mouse spleen cryosections with anti-mouse/rat Foxp3 antibody (FJK-16s). Image courtesy of Cintia De Paiva.
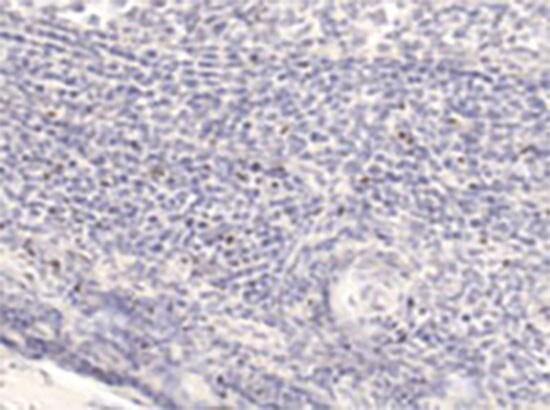
Figure 5. Human tonsillar tissue staining. Staining of human tonsillar tissue section with anti-human Foxp3 (PCH101). Image courtesy of Roger Sutmuller.
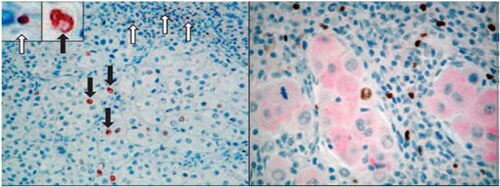
Figure 6. Lymphocyte and tumor cell staining. IHC staining of human spleen sections (left) and tumor (right) using the Foxp3 antibody on FFPE sections.
Related products
Clone information
Mouse Foxp3
Biller, BJ., et al. 2007. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. 116(1-2):69-78. [Intracellular staining for flow cytometry using FJK-16s in canine]
Sakaguchi, S. et al. 2005. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 202: 885-891. [Intracellular staining for flow cytometry using FJK-16s]
Fields, ML., et al. 2005. CD4+CD25+Regulatory T Cells Inhibit the Maturation but Not the Initiation of an Autoantibody Response. J. Immunol. 175: 4255 - 4264. [Intracellular staining for flow cytometry using FJK-16s]
Beyersdorf, S., et al. 2005. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 202: 445-55. [Intracellular staining for flow cytometry using FJK-16s (correction; not mFoxy)]
Anderson, M.S., et al. 2005. The cellular mechanism of Aire control of T cell tolerance. Immunity. 28: 227-39. [Intracellular staining for flow cytometry using FJK-16s]
Siegmund, R., et al. 2005. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 106: 3097-104. [Intracellular staining for flow cytometry using FJK-16s]
Wilson, M.S., et al. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 202: 1199-1212. [Intracellular staining for flow cytometry using FJK-16s]
Kretschmer, K., et al. 2005. Inducing and expanding regulatory T cells population by foreign antigen. Nat. Immunol. 6(12): 1219-1227. [Intracellular staining for flow cytometry using FJK-16s]
Human Foxp3 (PCH101)
Ahmadzadeh, M., et al. 2005. IL-2 Administration Increases CD4+CD25hiFoxp3+ Regulatory T Cells in Cancer Patients. Blood (Nov Epub). [Intracellular staining for flow cytometry using PCH101]
Crellin, NK., et al. 2005. Human CD4+ T Cells Express TLR5 and Its Ligand Flagellin Enhances the Suppressive Capacity and Expression of FOXP3 in CD4+CD25+ T Regulatory Cells. J. Immunol. 175(12): 8051-9. [Intracellular staining for flow cytometry using PCH101]
Hartwig, UF., et al. 2005. Depletion of alloreactive T cells via CD69: implications on antiviral, antileukemic and immunoregulatory T lymphocytes. Bone Marrow Transplant (Dec 5 Epub ahead of print). [Intracellular staining for flow cytometry using PCH101]
Lim, HW., et al. 2005. Cutting Edge: Direct Suppression of B Cells by CD4+CD25+ Regulatory T Cells. J. Immunol. 175: 4180 - 4183. [IHC of frozen sections using 236A/E; Intracellular staining for flow cytometry using PCH101]
Human Foxp3 (236A/E7)
Alvaro, T., et al. 2005. Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin. Cancer Res. 11(4):1467-73. [IHC paraffin using 236A/E7]
Roncador, G., et al. 2005. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 35: 1681-91. [IHC of paraffin sections using 236A/E; Intracellular staining for flow cytometry using 236A/E]
Roncador, G., et al. 2005. FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia (Epub ahead of print). [IHC paraffin sections using 236A/E7]
Wolf, D., et al. 2005. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. 11(23): 8326-31. [IHC paraffin using 236A/E7]
References
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057-1061.
- Lu, L., Barbi, J. & Pan, F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol 17, 703–717 (2017).
- Allan SE, Alstad AN, Merindol N, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16(1):194-202.
- Liechti T, Roederer M. OMIP-060: 30-Parameter Flow Cytometry Panel to Assess T Cell Effector Functions and Regulatory T Cells. Cytometry A. 2019;95(11):1129-1134.
- Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891-900.
- Presicce P, Moreno-Fernandez ME, Lages CS, Orsborn KI, Chougnet CA. Association of two clones allows for optimal detection of human FOXP3. Cytometry A. 2010;77(6):571-579.
- Schreiber TH. The use of FoxP3 as a biomarker and prognostic factor for malignant human tumors. Cancer Epidemiol Biomarkers Prev. 2007;16(10):1931-1934.
For Research Use Only. Not for use in diagnostic procedures.

