Search Thermo Fisher Scientific
Microbiome (Microbiota) Overview
In the human body, cells of microbial origin outnumber human cells ten to one and bacterial genes outnumber human genes one hundred-fold. This awareness has increased appreciation of the role microbes play in promoting human health and the effect perturbations in the symbiotic relationship between human cells and microbiota may have on diseases such as rheumatoid arthritis, diabetes, multiple sclerosis, and obesity.
Commensal microbiota
While epithelial cells are often viewed as the first line of defense against pathogenic insult, the commensal microbiota thriving on epithelial surfaces serves as an invisible barrier to invading pathogens. Symbiosis of non-pathogenic microbes prevents disease in a number of ways:
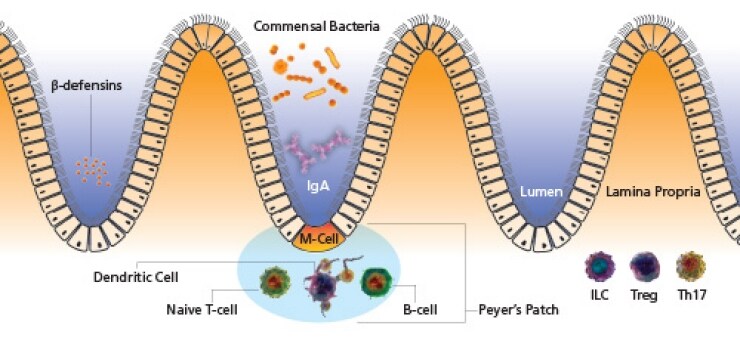
- Competition for adherence to the epithelia and mucosal surfaces as well as acidification of the microenvironment to prevent attachment
- Competition for nutrients
- Secretion of anti-microbial peptides
- Development of gut-associated lymphoid tissues (GALT)
When the commensal microbiota is disturbed as in the case of broad-spectrum antibiotic use, the resulting imbalance can lead to infection by opportunistic bacteria. Additionally, abnormal microbiota have been described in many inflammatory and autoimmune diseases (Crohn’s Disease, ulcerative colitis, inflammatory arthritis, type-1 diabetes, multiple sclerosis, etc.). For example, when mice are born under pathogen-free conditions with a complete lack of microbial colonization, severe immune defects are observed including: absence of Th17 T-cells, abnormal populations of mucosal B and T cells, diminished levels of IgG and IgA, and defective formation of secondary lymphoid organs in the gut.
Epithelia
Epithelial cells form the semi-impermeable mechanical barrier between body and outside world. Tight junctions between cells allow for an almost continuous layer of cells, preventing invasion by foreign microbes. Additionally, mucosal epithelia are armed with a vast array of anti-microbial peptides to help prevent and combat infection. Within the gut epithelia are specialized M cells that transport luminal antigens across the epithelium to dendritic cells in the lamina propria and Peyer’s patches.
Th17 cells
Th17 cells are a subset of CD4+ T-cells that can be found in intestinal lamina propria and gut-associated lymphoid tissues (GALT). Through the production of cytokines (IL-17A, IL-17F, IL-17AF, IL-22, and others), Th17 cells protect against infection by extracellular pathogens by recruiting neutrophils, promoting the secretion of anti-microbial peptides by epithelial cells, activating macrophages and other T-cell subsets, and by promoting B-cell survival, proliferation and germinal center formation. The composition of the gut microbiota has been shown to be crucial to Th17 cells as evidenced by the fact that Th17 cells are virtually absent in commensal bacteria, and can be reconstituted by the addition of specific commensal bacterial strains (e.g., segmented filamentous bacteria).
Treg cells
Similar to Th17 cells, the fate of Treg cells in the gut has been linked to the composition of intestinal microbiota. In this regard, as changes in the composition of the microbiota alters the ratio of Th17 to Treg cells. However, unlike Th17 cells, Treg development is relatively unaffected by the absence of a healthy microbial population. Some species of Clostridium have been shown to increase the percentage of Treg cells in the colon, where Treg cells likely act to suppress effector T-cell responses to the normal microbiota. It has been proposed that altering the microbiota to favor formation of Treg cells could serve as treatment for inflammatory autoimmune diseases such as Inflammatory Bowel Syndrome.
Microbiota references
Mathis D., Benoist C. (2011). Microbiota and autoimmune disease: the hosted self. Cell Host Microbe. 10, 297–30.
Hadis, U., Wahl, B., Schulz, O., Hardtke-Wolenski, M., Schippers, A., Wagner, N., Mu¨ ller, W., Sparwasser, T., Forster, R., and Pabst, O. (2011). Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246.
Littman D.R., Pamer E.G. (2011). Role of the Commensal Microbiota in Normal and Pathogenic Host Immune Responses. Cell Host Microbe. 10, 311–23.
Qin J, Li R, Raes J, Arumugam M, et. al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (7285):59–65.
Ivanov I. I., Littman D. R. (2010). Segmented filamentous bacteria take the stage. Mucosal Immunol. 3, 209–212. doi: 10.1038/mi.2010.3.
Wu, H.J., Ivanov, I.I., Darce, J., Hattori, K., Shima, T., Umesaki, Y., Littman, D.R., Benoist, C., and Mathis, D. (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells . Immunity 32, 815–827.
Gaboriau-Routhiau, V., Rakotobe, S., Le´cuyer, E., Mulder, I., Lan, A., Bridonneau, C., Rochet, V., Pisi, A., De Paepe, M., Brandi, G., et al. (2009). The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689.
For Research Use Only. Not for use in diagnostic procedures.